Medication
AI in Personal Nutrition Market is Expected to Reach $12.71 Billion by 2031 at 17.6% CAGR.

AI in the Food Market is unique
InsightAce Analytic Pvt. Ltd. announces the release of a market analysis report on «Global AI in Personalized Nutrition Market- (By Type (Machine Learning, Deep Learning, Natural Language, Computer Vision, etc.) Application (Diet Planning and Recommendations, Nutritional Testing), Individual Supplementation, Allergen and Sensitivity Testing, Health Care, etc.) By User By Provider, By Site, By Method, An Analysis of Industry Competition, Revenue and Speed to 2031.»
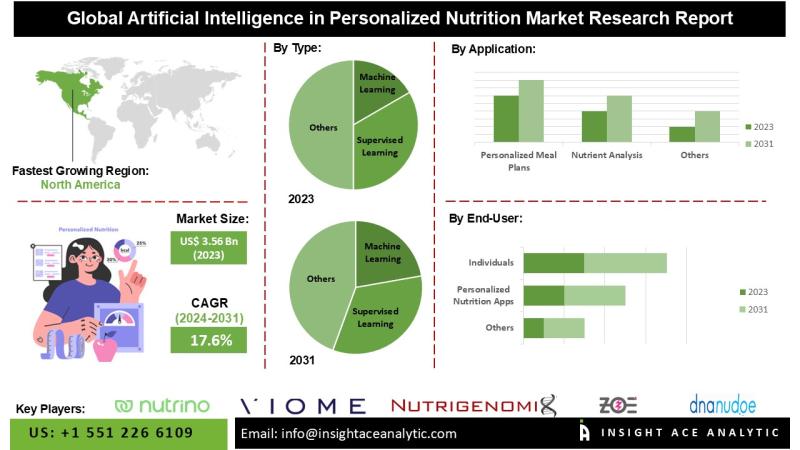
According to the latest research conducted by InsightAce Analytic, the Global AI in Nutrition Market is valued at $3.56 Bn in 2023, and is expected to reach $12.71 Bn in 2031, with a CAGR of 17.6% during conception. 2024-2031.
Get Free Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2691
AI in nutrition plays a key role in creating personalized strategies to meet individual nutritional needs, health goals and genetics. Al’s connection to nutrition has spawned a growing market, with new technologies and data-driven insights that provide dietary recommendations for consumers. Many methods have developed Al-based nutritional supplements, which are more nutritious
market expansion:
Genomic Profiling: The integration of Al and genetic analysis has enabled a deeper understanding of human genetics in specific health conditions and nutrient metabolism. By considering genomic data, Al can modify food recommendations to reduce the influence of genes on health outcomes.
List of Top Players in AI in Specialty Nutrition Market:
• Nutrino Health Ltd.
• DayTwo Ltd.
• Lumens
• Nutrigenomix Inc.
• Viome
• Foodvisor
• Good Foundation
• GenoPalate
• Practice
• Nutraceutical Association
• Nutrafol
• Zoe
• White
• FitGenie
• Level Foods Inc.
• Nutrition
• NutriAI
• NutrinoTech
• AIMEE Health
• NutriMe
• PreBiomics
• NutrEval
• NutriAdmin
• Proper nutrition
• NutriPredict
• The rest
Expertise, Just a Typing: https://caendly.com/insightaceanalytic/30min?month=2024-02
Market Dynamics:
Drivers:
Increasing consumer awareness about health and wellness is driving the demand for nutritional solutions. AI’s ability to analyze a wide range of data, including genetics, lifestyle and dietary patterns, greatly facilitates nutrition planning. Technological advances in AI and machine learning are improving the accuracy and effectiveness of these personalized recommendations. The increasing prevalence of chronic diseases and obesity is encouraging people to seek advice on the foods they are used to. In addition, growing investments in health technology startups and partnerships between technology companies and nutritionists are accelerating the growth of the market.
Problems:
Another major obstacle is privacy and data protection, as personalized nutrition relies on important health data that must be protected against fraud and misuse. In addition, the accuracy and reliability of AI algorithms are important, requiring extensive and continuous validation to ensure effective and safe food recommendations. Another challenge is integrating AI solutions with existing health and nutrition systems, which often require collaboration. In addition, consumer trust and acceptance of AI-driven nutritional advice remain obstacles, as individuals may question automated recommendations over traditional human knowledge. Finally, regulatory oversight is complex, with varying standards across regions, complicating global delivery of AI-driven personalized nutrition solutions.
Local Customs:
The North American AI in nutrition market is expected to register the largest market share in terms of revenue and is expected to grow at a high CAGR in the near future. This growth is driven by increasing consumer demand for personalized health solutions, advancements in AI technology, and increasing awareness of the benefits of nutrition plans. The region’s strong technological infrastructure also supports this market expansion. In addition, Europe has a major share due to its advanced healthcare services and growing consumer demand for personalized nutrition solutions. This area benefits from strong research and development activities and a growing emphasis on health and wellness. European countries are using AI to improve nutrition planning, making nutrition more accessible and effective for different populations.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2691
Latest Update:
• In 2022, DNAfit acquired Nutrigenomix, a company that provides nutrition and lifestyle advice based on genetic testing.
probiotic supplement designed to improve gut health.
AI Segmentation in the Nutrition Market is unique
AI in the Specialty Nutrition Market- By Type
• Machine Learning
o Formal Education
o Unexpected Learning
o Reinforcement Learning
• Deep Learning
o Convolutional Neural Networks (CNN)
o Recurrent Neural Networks (RNN)
o Generative Adversarial Networks (GAN)
o Changes
• Natural Language Processing (NLP)
o Evaluation of emotions
o Language Translation
o Chatbots and Virtual Assistants
• Computer Vision
o Group of Pictures
o Finding something
o Face Recognition
o Video Analysis
AI in the Specialty Nutrition Market – Please
• Meal Planning and Recommendations
o Individual Meal Plans
o Recipe Suggestions
o Benefits of Dietary Supplements
• Energy Analysis
o Identifying Malnutrition
o Recommendations on Food
o Energy Development
• Exclusive contribution
o Additional Recommendations
o Recommendations for rates and timing
o Tracking and monitoring
• Allergen and Sensitivity Testing
o Allergen Information
o Other ingredient suggestions
o Dietary restrictions
• Health care
o Analysis of Biometric Data
o Real-time health feedback
o Acceptance of Behavior
AI in the Specialty Nutrition Market – By User
• Individuals
o Nutritional supplements
o Smart Devices and Wearables
• Fitness and Wellness Centers
o Trainers and Coaches
o Food Services
• Health care providers
o Hospitals and clinics
o Telehealth tools
• Food and Beverage Industry
o Food Manufacturers
o Food and Beverage Services
AI in the Personal Nutrition Market Provider-by-Provider
• Start-up and small companies
o Al-Powered Nutrition Startups
o New Tech solutions
• Established Tech Companies
o Tech giants
o Al Platform Providers
• Health and Wellness Organizations
o Hospitals and Medical Centers
o Health centers and institutions
AI in the Independent Food Market – By Region
North America-
• US
• Canada
• Mexico
Europe-
• Germany
• UK
• France
• Italy
• Spain
• In another part of Europe
Asia-Pacific
• China
• Japan
• India
• South Korea
• Southeast Asia
• Other Asia Pacific regions
Latin America-
• Brazil
• Argentina
• Other Latin American regions
Middle East & Africa-
• GCC countries
• South Africa
• Other areas in the Middle East and Africa
Empower Decision Making with 180 Pages Full Report @ https://www.insightaceanalytic.com/buy-report/2691
About Us:
InsightAce Analytic is a market research and consulting firm that helps clients make strategic decisions. Our qualitative and quantitative market intelligence solutions provide market demand awareness and competitive intelligence to grow businesses. We help clients gain competitive advantage by identifying untapped markets, evaluating new and competitive technologies, segmenting potential markets and repositioning products. Our expertise is in providing integrated and customized market intelligence reports with in-depth analysis containing valuable market data in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Phone: +1 551 226 6109
Asia: +91 79 72967118
[email protected]
This announcement was published on OpenPR.
#Personal #Nutrition #Market #Expected #Reach #Billion #CAGR
Medication
5WPR Expands Health and Wellness Divide with New Focus on Functional Nutrition

Strengthening Capacity to Support Growing Demand in the Functional Food Sector
NEW YORK, December 3, 2024 /PRNewswire/ — 5WPR, one of America’s leading and largest independent PR firms, is proud to announce the expansion of its Health & Wellness division to include focusing on Functional Nutrition. This strategic move reflects 5W’s commitment to staying ahead of industry trends and evolving its services to meet the needs of this rapidly changing market.
With increasing consumer interest in functional foods, brands are seeking expert guidance to differentiate themselves in a competitive market. In response, 5WPR has expanded its services to cover key areas within the nutrition industry, including gut health, supplements and functional drinks, performance and sports nutrition, protein powder, probiotics, weight management, collagen peptides, dietary powder, functional mushrooms. , nutrition, and pet health.
Functional nutrition has become an influence in the field of health and wellness, fueled by increased consumer awareness and demand for products that provide more than basic nutritional benefits. According to the latest data from Open PR, gut health products alone are estimated to grow by approximately 8% annually between 2024 and 2031. Additionally, the Food Institute is report that e-commerce has emerged as the fastest growing channel for supplements in 2023, an increase of 9.5%, highlighting the importance of a strong online presence. Performance nutrition has also seen significant growth, with categories such as hydration and electrolytes increasing by 52.2%, and creatine sales increasing by an impressive 114.4%, according to the New Hope Network.
«The functional nutrition market is not only growing; it is defining the health and wellness industry,» he said. Ilisa WirginManaging Partner and EVP of 5WPR’s Health & Wellness and Beauty division. «Consumers are looking for products that improve their overall well-being, improve performance, and support recovery, which makes it an exciting and exciting segment. Our expanded focus will help us helping customers in this area to grow their product profiles, align with their target audience, and deliver meaningful results.»
5WPR’s track record in health and wellness includes working with some of the most recognized brands in fitness, nutrition, and personal care. The agency’s expertise in media relations, influencer partnerships, thought leadership, digital strategy, and affiliate marketing ensures that clients in the nutrition space receive structured, comprehensive, and measurable support.
#5WPR #Expands #Health #Wellness #Divide #Focus #Functional #Nutrition
Medication
Lady Gabriella’s husband ‘killed himself after taking antidepressants’

Your support helps us tell the story
From reproductive rights to climate change to Big Tech, The Independent is breaking new ground as the story unfolds. Whether it’s investigating Elon Musk’s pro-Trump PAC funding or presenting our latest article, ‘The A Word’, which shines a light on American women fighting for reproductive rights, we know how How important is clarity in messages.
At such a critical time in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to talk to both sides of the story.
The Independent is trusted by Americans across all political lines. And unlike many other quality publications, we choose not to block Americans from reporting and reviewing through paywalls. We believe that quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.
Lady Gabriella Kingston’s husband took his own life after taking anti-depressants prescribed by his doctor, a coroner has said.
Thomas Kingston, who married Prince and Princess Michael of Kent’s daughter at Windsor Castle in 2019, died of a head injury and a gun was found next to his body on March 25 at his parents’ home in the Cotswolds.
At an inquest into her death held at Gloucestershire Coroner’s Court on Tuesday, Lady Gabriella, 43, asked people to be warned about the side effects of drugs used to treat mental health conditions or she feared people would many may die.
The inquest was told that the 45-year-old fundraiser had been given sertraline, a drug used to treat depression, and zopiclone, a sleeping pill, by a doctor at the Royal Mews Surgery, Buckingham Palace’s staff-run practice. of the palace. , after complaining of trouble sleeping after stress at work.
Mr Kingston went back to surgery saying they were not making him feel better, and his doctor switched him from sertraline to citalopram, a similar drug.

In the first days of his death, Mr. Kingston had stopped taking medication, and toxicology tests showed caffeine and a small amount of zopiclone in his system.
In a statement read out by Katy Skerrett, chief minister of Gloucestershire, Lady Gabriella, 43, said: «[Work] it has been a challenge for her over the years but I highly doubt it would have made her suicidal, and she seemed to have improved a lot.
If there was something that was bothering him, I’m sure he would have said that he was struggling a lot.
The fact that he committed suicide in the home of his beloved parents suggests that the decision was the result of a sudden impulse.
He said he believed her death «may have been precipitated» by an adverse reaction to medication she had started, and then stopped, taking in the weeks leading up to her death.
«In the absence of any evidence of propensity, it seems very likely to me that she had a bad reaction to the pills that made her take her own life,» Lady Gabriella said.

«I believe that anyone who takes pills like these should be warned about the side effects to prevent future deaths.
If this can happen to Tom, it can happen to anyone.
In his final weeks, Mrs Gabriella said, her husband «seemed normal», except for the morning after he first took zopiclone, which she said made him seem «he he’s a bit confused».
In his statement, he described their marriage as one of «deep love and trust» and said he never gave any suicidal thoughts to himself or others.
He also said he was deeply affected by his friend’s suicide and «the negative impact it had on other people’s loved ones».
Miss Gabriella, whose wedding to Mr Kingston included Queen Elizabeth II and the late Duke of Edinburgh among the guests, wept as she sat in the coroner’s court as her statement was read.

Wrapping up the story, Ms Skerrett said: «Mr Kingston took his own life with a self-inflicted gunshot wound to the head.
«The evidence of his wife, family and business partner all support the lack of intent to kill himself.
He was suffering from the side effects of the medicine he had just been given.»
He said he intends to make a report on the prevention of future deaths, which will be sent to medical societies.
Mr. Kingston’s father, William Martin Kingston, wept as he described finding his son in the locked bathroom of the annexe, having used a doorknob to break the door.
He told the court that his son has always had a strong, unstable character, as he previously suffered from pain that left him needing help to climb the stairs.
She told the coroner that before her son’s death there did not appear to be any evidence of suicide, and there was no will, describing the process as «reckless.» very» who was «out of shape».
Dr David Healy, a psychiatrist who gave evidence at the hearing, said zopiclone could also cause anxiety, while sertraline and citalopram were both serotonin reuptake inhibitors (SSRIs), and while exactly the same.
Dr Healy said Mr Kingston’s complaints that sertraline continued to cause him anxiety was a sign SSRIs «didn’t suit him», and he should not have been prescribed the same thing again.
He said the guidelines and labels for SSRIs were not clear enough about using the drug first, or what the effect would be when switching from one to another.
He said: «We need a clearer statement that these drugs can cause people to kill themselves who would otherwise not kill themselves.
Speaking to the doctor, Martin Porter, the family’s adviser, said: «The family is not to blame (her doctor) Dr Naunton Morgan, he worked like a good doctor.
But the question is whether there is enough advice to doctors about SSRIs.
#Lady #Gabriellas #husband #killed #antidepressants
Medication
AlayaCare’s Cloud-Based EHR Platform Onboards Home Care Benefits of the Home Care Center

AlayaCare’s Cloud-Based EHR Platform Onboards Home Care Benefits of the Home Care Center
NEW YORK, Dec. 03, 2024 (GLOBE NEWSWIRE) — AlayaCare, a global technology platform for home and community care, is pleased to announce today the successful implementation of Care Advantage, Inc.
Care Advantage, Inc. is the largest independent home health provider in the mid-Atlantic. The company provides personal services, home care and home health services throughout Virginia, Maryland, Delaware, North Carolina and Washington DC.
Care Advantage’s leadership recognized AlayaCare’s ability to provide a risk-free, user-friendly solution that could grow with the company. The decision to collaborate with AlayaCare was made after an extensive selection process that considered the opinions of experts from its internal teams, emphasizing the importance of a holistic approach to choosing a technology partner.
«We were looking for a partner, not just a vendor, who could help us build and transform our system over time,» said Tim Hanold, CEO of Care Advantage. «AlayaCare’s commitment to working together and creating a true partnership were key factors in our final decision.»
Despite the challenges presented by the published timeline and coverage of more than 50 locations, both teams worked closely to customize the system to meet Care Advantage’s needs.
«Implementing a system of this size in such a short period of time was no small feat,» said Jaron Clay, VP of Integrations. «The speed of the rollout was only possible with good coordination and both groups are committed to having Care Advantage running smoothly and feeling the benefits of the new system as soon as possible.»
The move to AlayaCare underscores Care Advantage’s commitment to innovation and excellence in home care, ensuring clients and their families receive the best possible care and support. As a highly data-driven and growth-focused company, the flexibility of AlayaCare’s platform enables large operations to enter new markets while maintaining a high level of customer care. own and create new opportunities for its custodians.
«There is a shared understanding and vision between us and the AlayaCare team,» said Tim Hanold. «They support our unwavering commitment to excellence in the classroom and are part of the reason we chose them. We feel this is the platform to take us to that level. We are delighted to have AlayaCare as a partner our technology,» he concluded. «Together, we’re setting new standards for home care.»
#AlayaCares #CloudBased #EHR #Platform #Onboards #Home #Care #Benefits #Home #Care #Center
-

 Health care10 meses ago
Health care10 meses agoIf you need a break to shut down during the holidays, you’re not alone, new research finds | CNN
-

 Medication10 meses ago
Medication10 meses agoPCOR awards $156 million in new patient-centered health research
-

 Medication10 meses ago
Medication10 meses ago5WPR Expands Health and Wellness Divide with New Focus on Functional Nutrition
-

 Health care10 meses ago
Health care10 meses agoGlobal Sports and Food Aging Food and Beverage Market 2024 to 2031
-

 Mental health10 meses ago
Mental health10 meses agoText message reminders fail to boost long-term medication use
-

 Mental health10 meses ago
Mental health10 meses agoAll we want for Christmas is some time alone, a new survey says. Here’s how to get more for yourself this holiday season
-

 Medication10 meses ago
Medication10 meses agoNew insights into anti-tuberculosis drugs during pregnancy
-

 Health care10 meses ago
Health care10 meses agoPilot program brings culinary medicine to PA students




